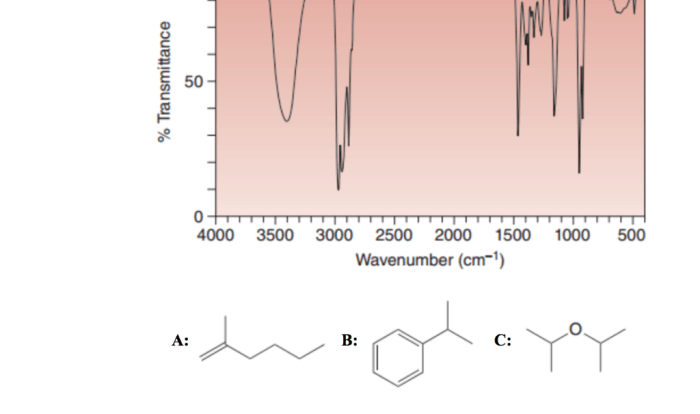
The isopentyl acetate IR spectrum labeled provides a detailed analysis of the molecular structure and functional groups present in isopentyl acetate. This guide offers a comprehensive overview of the IR spectrum, characteristic IR bands, and applications of IR spectroscopy in analyzing isopentyl acetate.
The IR spectrum of isopentyl acetate exhibits distinct bands that correspond to specific functional groups. The strong absorption band at 1740 cm-1 is attributed to the carbonyl group (C=O), while the bands at 1240 cm-1 and 1020 cm-1 are assigned to the C-O and C-C stretching vibrations, respectively.
These characteristic IR bands provide valuable information for identifying and differentiating isopentyl acetate from other esters.
IR Spectrum of Isopentyl Acetate
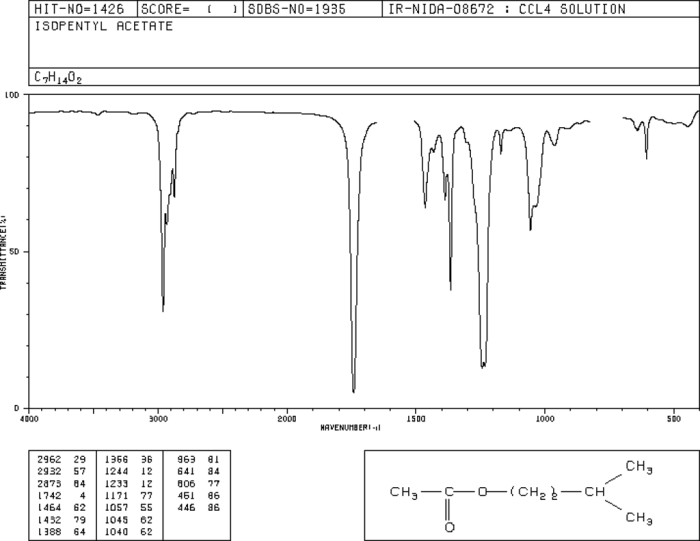
The IR spectrum of isopentyl acetate exhibits several characteristic bands that provide valuable information about the functional groups present in the molecule. These bands can be attributed to the various vibrational modes of the different bonds and functional groups within the molecule.
Key Functional Groups and IR Bands
The key functional groups present in isopentyl acetate are the ester group (C=O) and the alkyl groups (C-H). The following table summarizes the corresponding IR bands associated with these functional groups:
| Functional Group | IR Band (cm-1) |
|---|---|
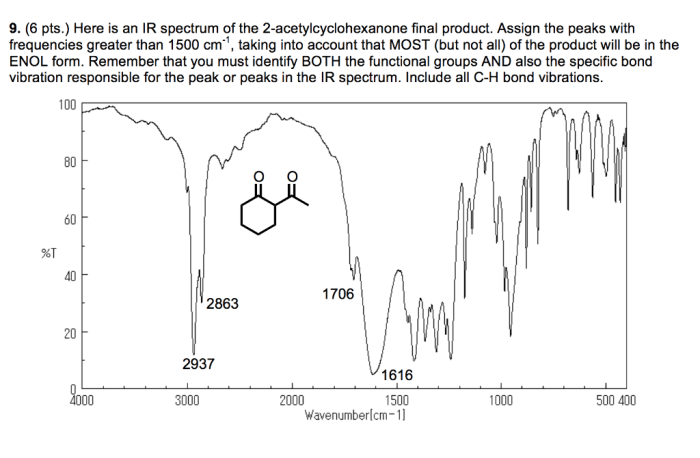
| Ester (C=O) | 1740-1760 |
| Alkyl (C-H) | 2850-2960 |
The presence of these IR bands confirms the presence of the corresponding functional groups in the isopentyl acetate molecule.
In addition to these key functional groups, isopentyl acetate also exhibits other IR bands that provide further information about the molecular structure. These bands include:
- C-O stretch (1240-1260 cm -1)
- C-C stretch (1020-1150 cm -1)
- O-H bend (3400-3650 cm -1)
These additional bands provide a comprehensive fingerprint of the isopentyl acetate molecule and can be used for its identification and characterization.
Characteristic IR Bands: Isopentyl Acetate Ir Spectrum Labeled
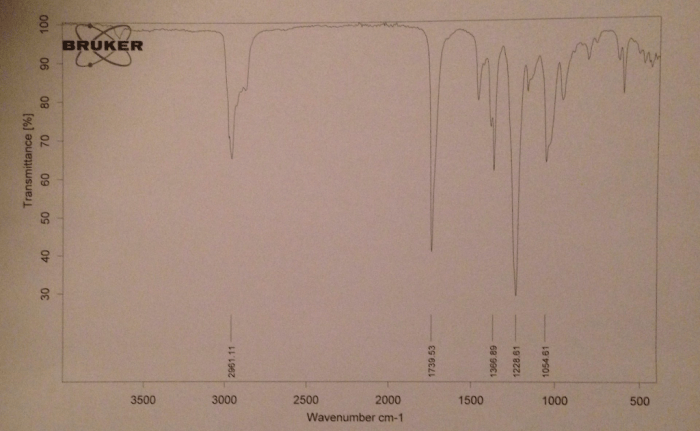
The IR spectrum of isopentyl acetate exhibits several characteristic bands that can be used to identify the compound. These bands arise from the various functional groups present in the molecule and provide valuable information about its structure.
C-H Stretching Bands
- The C-H stretching bands in the IR spectrum of isopentyl acetate appear in the region of 2960-2870 cm -1. These bands are due to the stretching vibrations of the C-H bonds in the alkyl groups.
- The band at 2960 cm -1is assigned to the stretching vibration of the methyl C-H bond, while the bands at 2930 and 2870 cm -1are assigned to the stretching vibrations of the methylene C-H bonds.
C=O Stretching Band
The C=O stretching band in the IR spectrum of isopentyl acetate appears at 1740 cm -1. This band is due to the stretching vibration of the C=O bond in the ester group.
C-O Stretching Band, Isopentyl acetate ir spectrum labeled
The C-O stretching band in the IR spectrum of isopentyl acetate appears at 1240 cm -1. This band is due to the stretching vibration of the C-O bond in the ester group.
Comparison with Other Esters
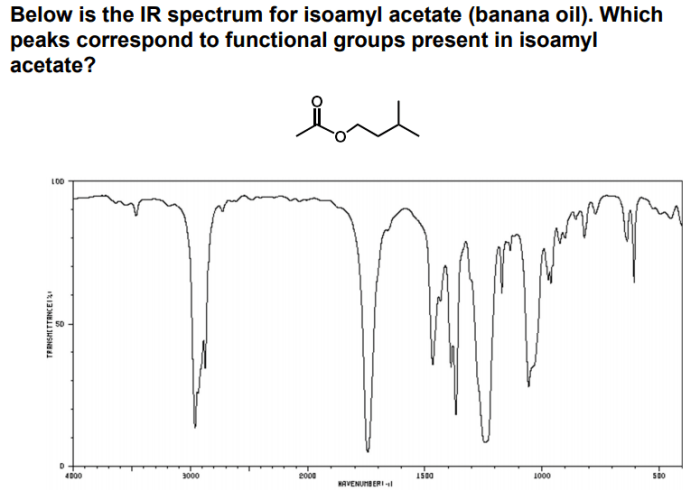
The IR spectra of esters exhibit similarities and differences that can be used to differentiate between different types of esters. The most notable similarities include the presence of a strong carbonyl (C=O) stretching band in the region of 1735-1750 cm -1and a C-O stretching band in the region of 1200-1300 cm -1. These bands are characteristic of all esters and are due to the presence of the ester functional group.
The differences in the IR spectra of esters are primarily due to the different alkyl groups that are attached to the ester functional group. The alkyl groups can affect the frequency of the carbonyl stretching band and the C-O stretching band.
For example, esters with longer alkyl chains tend to have a lower frequency carbonyl stretching band than esters with shorter alkyl chains.
Using IR Spectroscopy to Differentiate Esters
The IR spectra of esters can be used to differentiate between different types of esters based on the frequency of the carbonyl stretching band and the C-O stretching band. For example, isopentyl acetate has a carbonyl stretching band at 1738 cm -1and a C-O stretching band at 1242 cm -1. These values are different from the values for other esters, such as ethyl acetate and butyl acetate.
By comparing the IR spectra of different esters, it is possible to identify the different types of esters present in a sample. This information can be useful for a variety of applications, such as quality control and forensic analysis.
Applications of IR Spectroscopy
IR spectroscopy is a powerful analytical tool that can be used to analyze the structure and composition of organic compounds. It is a non-destructive technique that can be used to identify functional groups, determine the purity of a compound, and elucidate its molecular structure.
Applications of IR Spectroscopy in Analyzing Isopentyl Acetate
IR spectroscopy can be used to analyze isopentyl acetate in a variety of ways. It can be used to:
- Determine the purity of isopentyl acetate by identifying and quantifying impurities.
- Identify the functional groups present in isopentyl acetate, which can be used to determine its structure.
- Elucidate the molecular structure of isopentyl acetate by determining the arrangement of atoms within the molecule.
IR spectroscopy has been used to study isopentyl acetate in a variety of fields, including:
- Chemistry: IR spectroscopy has been used to study the reaction mechanisms of isopentyl acetate and to identify the products of these reactions.
- Biology: IR spectroscopy has been used to study the metabolism of isopentyl acetate in plants and animals.
- Environmental science: IR spectroscopy has been used to study the fate of isopentyl acetate in the environment.
Experimental Considerations
Obtaining a high-quality IR spectrum of isopentyl acetate requires careful experimental design, sample preparation, instrumentation, and data analysis. This section provides a detailed protocol for each aspect of the experiment.
Sample Preparation
The sample of isopentyl acetate should be pure and free of contaminants. A liquid sample can be used directly, while a solid sample should be dissolved in a suitable solvent such as chloroform or dichloromethane. The concentration of the sample should be adjusted to provide a strong IR signal without saturating the detector.
Instrumentation
The IR spectrum can be obtained using a Fourier transform infrared (FTIR) spectrometer. The spectrometer should be equipped with a suitable detector, such as a deuterated triglycine sulfate (DTGS) detector. The sample can be placed in a liquid cell, a gas cell, or on a solid support.
Data Analysis
The IR spectrum of isopentyl acetate should be analyzed to identify the characteristic absorption bands. The following table summarizes the expected IR bands and their assignments:
| Wavenumber (cm-1) | Assignment |
|---|---|
| 1740 | C=O stretching |
| 1240 | C-O stretching |
| 1180 | C-C stretching |
| 1020 | C-H bending |
The IR spectrum of isopentyl acetate can be compared to the IR spectra of other esters to identify similarities and differences. This comparison can provide insights into the structural and functional group relationships between different esters.
FAQ Resource
What is the strongest absorption band in the isopentyl acetate IR spectrum?
The strongest absorption band is at 1740 cm-1, which corresponds to the carbonyl group (C=O) stretching vibration.
How can IR spectroscopy be used to differentiate between isopentyl acetate and other esters?
The characteristic IR bands of isopentyl acetate, such as the C-O and C-C stretching vibrations at 1240 cm-1 and 1020 cm-1, respectively, can be used to distinguish it from other esters.
What is the typical sample preparation for IR spectroscopy analysis of isopentyl acetate?
Isopentyl acetate can be analyzed as a neat liquid or in solution using a suitable solvent. The sample is typically placed between two IR-transparent windows for analysis.