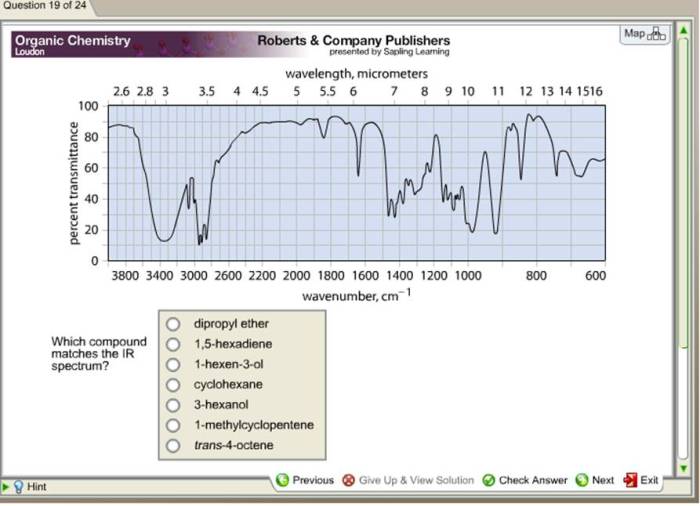
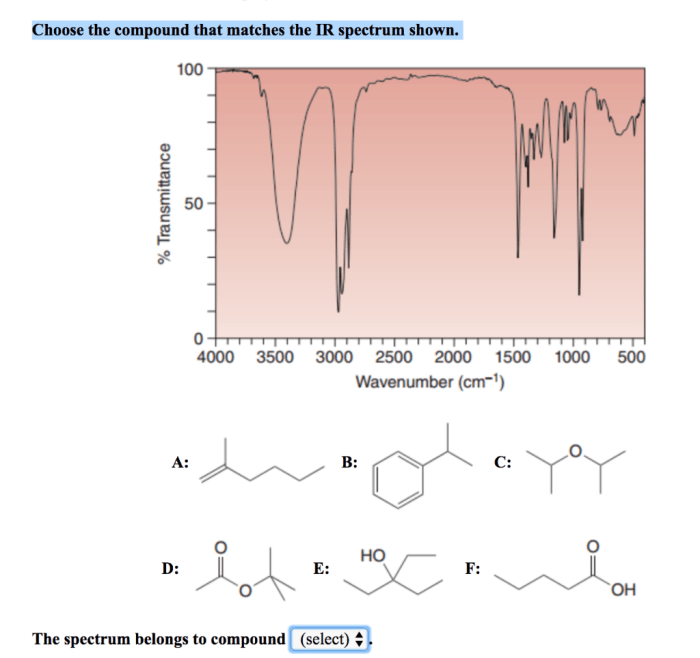
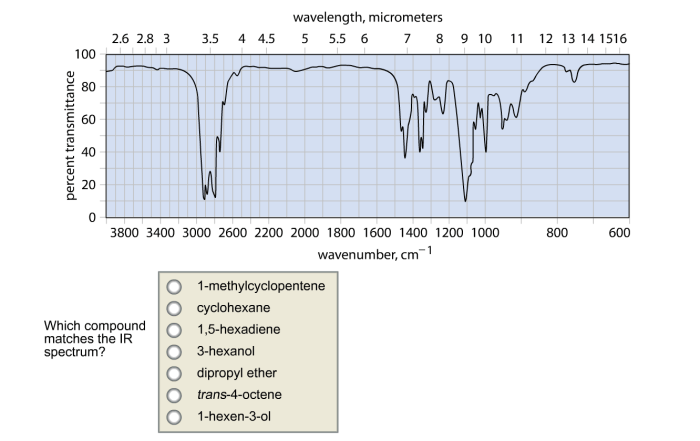
Which compound matches the ir spectrum – Delving into the realm of infrared (IR) spectroscopy, this exploration unveils the profound connection between molecular vibrations and their characteristic IR absorption frequencies. By harnessing this knowledge, scientists embark on a quest to identify compounds with remarkable precision, deciphering their intricate molecular structures.
Through the meticulous analysis of IR spectra, we unravel the symphony of functional groups that orchestrate a compound’s chemical identity. Armed with a comprehensive table of absorption frequencies, we decode the molecular blueprint, revealing the presence of specific functional groups that resonate at distinct frequencies.
Functional Group Identification
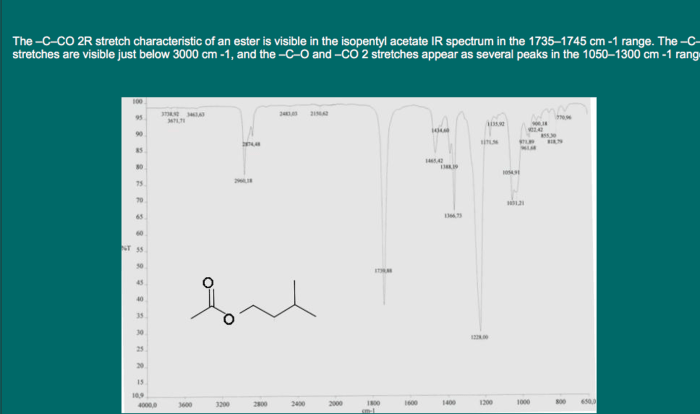
IR spectroscopy provides valuable information about the functional groups present in a compound. Each functional group has characteristic absorption frequencies in the IR spectrum. By analyzing the IR spectrum and comparing it to a table of known functional group frequencies, it is possible to identify the functional groups present in the compound.
Common Functional Groups and Their IR Absorption Frequencies, Which compound matches the ir spectrum
| Functional Group | IR Absorption Frequency (cm-1) |
|---|---|
| O-H (alcohol) | 3600-3700 |
| C=O (ketone) | 1700-1750 |
| C-N (amine) | 1250-1350 |
| C-O (ether) | 1050-1200 |
Structural Determination: Which Compound Matches The Ir Spectrum
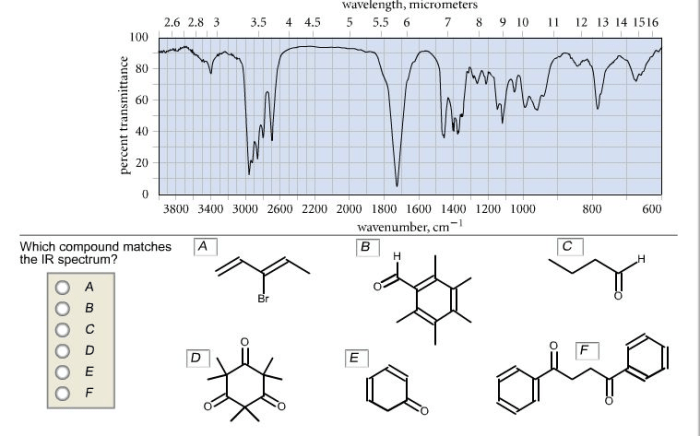
IR spectroscopy can also be used to determine the molecular structure of a compound. By analyzing the IR spectrum, it is possible to identify characteristic absorption bands that correspond to specific structural features. For example, the presence of a C-H stretching vibration at 2900-3000 cm -1indicates the presence of an aliphatic C-H bond.
Characteristic Absorption Bands and Structural Features
| Absorption Band (cm-1) | Structural Feature |
|---|---|
| 3300-3500 | O-H stretching (alcohol) |
| 2900-3000 | C-H stretching (aliphatic) |
| 1700-1750 | C=O stretching (ketone) |
| 1250-1350 | C-N stretching (amine) |
FAQ
How does IR spectroscopy aid in identifying compounds?
IR spectroscopy provides a unique fingerprint for each compound, allowing for precise identification based on the characteristic absorption frequencies of its functional groups.
What is the significance of functional group identification in IR spectroscopy?
Functional group identification serves as a crucial step in determining the molecular structure of a compound, as specific functional groups exhibit characteristic IR absorption frequencies.
How can IR spectroscopy be employed to determine molecular structure?
By analyzing the IR spectrum, scientists can identify characteristic absorption bands that correspond to specific structural features, enabling the deduction of the molecular structure.