Embark on an enlightening journey with the polyatomic ions worksheet answer key, your definitive guide to deciphering the complexities of these multifaceted ions. Dive into a comprehensive exploration of their significance, nomenclature, and practical applications, unlocking a deeper understanding of chemical reactions and the world around us.
This meticulously crafted worksheet provides a wealth of questions and answers, empowering you to master the identification, charge determination, and formula writing of polyatomic ions. Delve into real-world scenarios where these ions play pivotal roles, from maintaining electrolyte balance in living organisms to facilitating industrial processes and safeguarding our environment.
Polyatomic Ions Worksheet: Polyatomic Ions Worksheet Answer Key
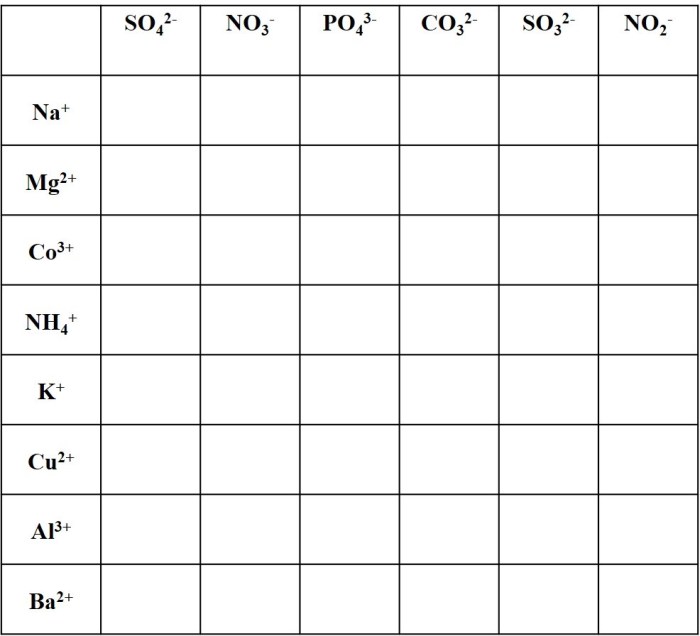
Polyatomic ions are groups of atoms that carry a net electrical charge and behave as a single unit within chemical reactions. They are composed of two or more elements, typically a metal and a nonmetal, and are named according to the elements present and their relative proportions.
Polyatomic ions play a crucial role in chemical reactions, as they can alter the properties of compounds and participate in various chemical processes. Understanding their behavior and nomenclature is essential for comprehending chemical reactions and predicting their outcomes.
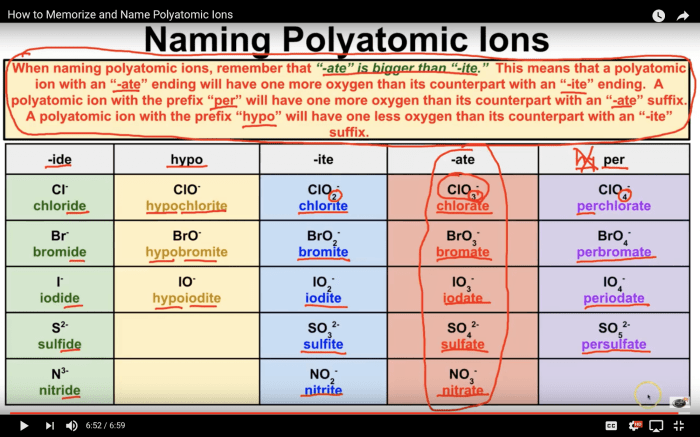
Naming Conventions for Polyatomic Ions, Polyatomic ions worksheet answer key
- The name of a polyatomic ion typically ends with the suffix “-ate” or “-ite”.
- The suffix “-ate” indicates that the ion contains the maximum number of oxygen atoms possible for the given element.
- The suffix “-ite” indicates that the ion contains one less oxygen atom than the “-ate” ion.
- Prefixes such as “per-” and “hypo-” may be used to indicate the presence of additional or fewer oxygen atoms, respectively.
Clarifying Questions
What is the definition of a polyatomic ion?
A polyatomic ion is a group of atoms that carry an electric charge and behave as a single unit within a chemical compound.
How are polyatomic ions named?
Polyatomic ions are named based on the elements they contain and their charge. The suffix “-ate” is used for ions with a negative charge, while “-ite” is used for ions with a lower negative charge.
What are some examples of polyatomic ions?
Common polyatomic ions include hydroxide (OH-), nitrate (NO3-), sulfate (SO42-), and carbonate (CO32-).