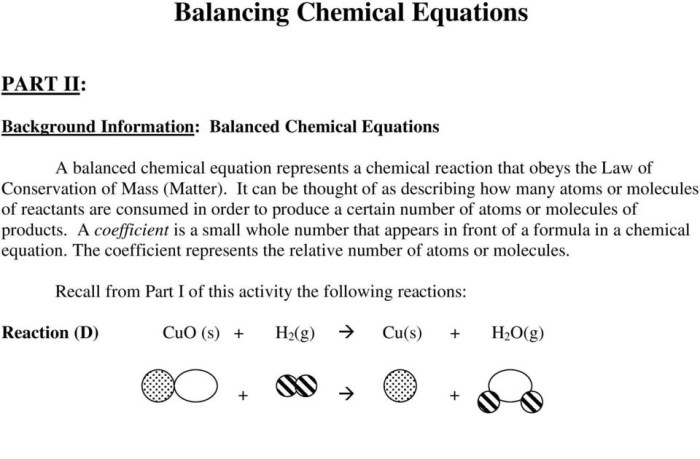
Embark on an enlightening journey into the realm of chemistry with our comprehensive guide to “Number of Atoms by Formula Worksheet Answers.” This essential resource empowers students to unravel the mysteries of chemical formulas, unlocking the secrets to determining the number of atoms within complex molecular structures.
Delve into the intricacies of chemical formulas, unraveling the significance of subscripts and coefficients, and master the art of organizing data with precision and clarity.
As we delve deeper into the topic, we will explore the factors that influence the number of atoms in a formula, shedding light on empirical and molecular formulas. By the end of this exploration, you will possess a profound understanding of the “Number of Atoms by Formula Worksheet,” enabling you to confidently tackle any chemical formula that comes your way.
Number of Atoms by Formula Worksheet
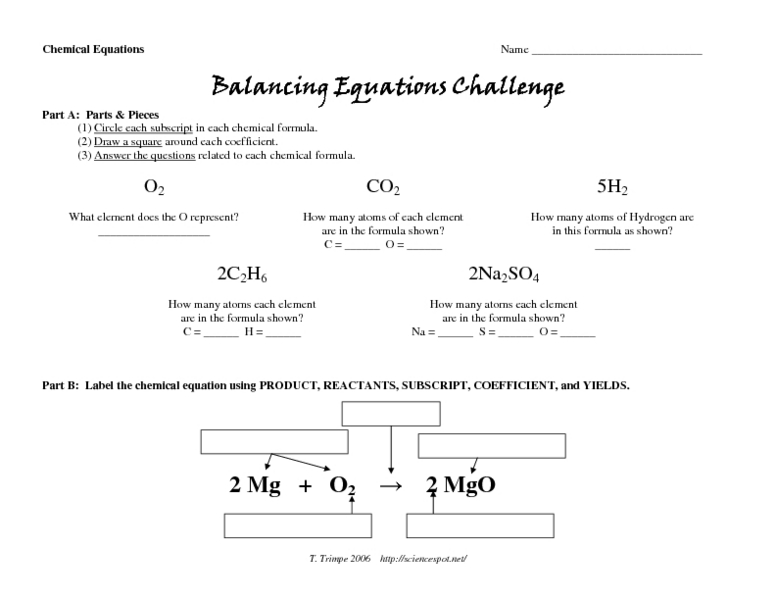
The “Number of Atoms by Formula Worksheet” is a tool designed to help students determine the number of atoms of each element present in a chemical formula. It provides a structured approach to understanding chemical formulas and their composition.
The worksheet typically includes a table with columns for each element and rows for each chemical formula. Students are expected to identify the chemical formulas, determine the number of atoms for each element, and organize the data in the table.
Identifying Chemical Formulas, Number of atoms by formula worksheet answers
Chemical formulas represent the composition of chemical compounds using symbols and numbers. Each symbol represents an element, and the subscripts indicate the number of atoms of that element present in the compound.
For example, the chemical formula H 2O represents water, which contains two hydrogen atoms (H) and one oxygen atom (O).
Coefficients, which are numbers placed before chemical formulas, indicate the number of molecules of that compound present. For example, 2H 2O represents two molecules of water.
Determining the Number of Atoms
To determine the number of atoms in a chemical formula, follow these steps:
- Identify the chemical formula.
- Locate the element of interest in the periodic table.
- Multiply the subscript of the element by the coefficient (if present).
For example, to determine the number of oxygen atoms in 2H 2O, multiply the subscript of oxygen (1) by the coefficient (2): 2 x 1 = 2 oxygen atoms.
Organizing the Data
Organize the number of atoms for each element in a table using HTML table tags:
| Element | Number of Atoms |
|---|---|
| Hydrogen | 2 |
| Oxygen | 1 |
This table clearly presents the number of atoms for each element in the chemical formula.
Additional Considerations
Factors that can affect the number of atoms in a chemical formula include:
- Empirical formulas vs. molecular formulas
- Ionic compounds
The “Number of Atoms by Formula Worksheet” has limitations and may not account for all these factors.
Helpful Answers: Number Of Atoms By Formula Worksheet Answers
What is the purpose of the “Number of Atoms by Formula Worksheet”?
The “Number of Atoms by Formula Worksheet” is designed to guide students in determining the number of atoms of each element present in a given chemical formula.
How do I determine the number of atoms in a chemical formula?
To determine the number of atoms in a chemical formula, multiply the subscript of each element by the coefficient preceding the formula.
What is the difference between an empirical formula and a molecular formula?
An empirical formula represents the simplest whole-number ratio of elements in a compound, while a molecular formula indicates the actual number of atoms of each element in a molecule.